Giampouras, M., Garrido, C. J., Zwicker, J., Vadillo, I., Smrzka, D., Bach, W., … & García-Ruiz, J. M. (2019). Geochemistry and mineralogy of serpentinization-driven hyperalkaline springs in the Ronda peridotites. Lithos, 350, 105215.
We present a detailed study of the water geochemistry, mineralogy and textures in serpentinization-related hyperalkaline springs in the Ronda peridotites. Ronda waters can be classified into hyperalkaline fluids and river waters that are broadly similar to Ca2+–OH– and Mg2+–HCO3– water types described in serpentinite-hosted alkaline springs elsewhere. At the discharge sites of the fluids (fractures or human made outlets) and ponds along the fluid flow paths, the fluids are hyperalkaline (10.9 < pH < 12) and characterized by low Mg and high Na, K, Ca, and Cl– concentrations. River waters, occurring near the spring sites, are mildly alkaline (8.5 < pH < 8.9) and enriched in Mg and DIC compared to Na, K, Ca and Cl–. The chemistry of Ronda Mg–HCO3 river waters is likely due to the hydrolysis of ferromagnesian peridotite minerals in equilibrium with the atmosphere by infiltrated meteoric water and shallow groundwater in the serpentinized peridotite. The Ronda Ca–OH hyperalkaline fluids are generated by the combination of low temperature serpentinization reactions from infiltrated surface Mg–HCO3 river waters —or Ca–HCO3 waters from near karst aquifers— and deep carbonate precipitation isolated from atmospheric CO2. Mass balance calculations indicate that the weathering of Ca-bearing peridotite silicates, such as diopside, is a feasible source of Ca in Ronda Ca–OH hyperalkaline fluids; however, it requires steady-state dissolution rates substantially greater than those determined experimentally.
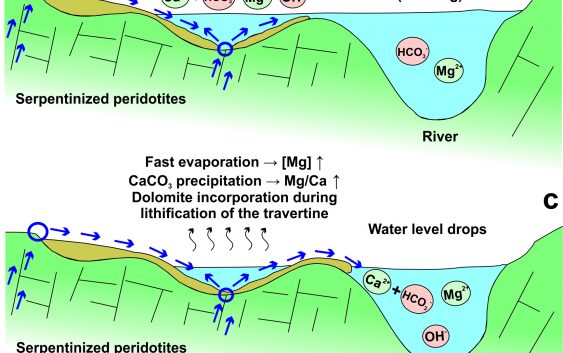
Travertine, crystalline crusts and sediment deposits comprise the types of solid precipitates observed in Ronda hyperalkaline spring sites. Calcite, aragonite, dolomite and Mg-Al-rich clays are the main mineral phases identified in the spring sites. As illustrated in the Baños del Puerto spring site, (i) calcite-dominated precipitation is due to uptake of atmospheric CO2 by Ca–OH hyperalkaline fluids during discharge, and (ii) aragonite-dominated precipitation is due to mixing of Ca–OH hyperalkaline fluids with Mg–HCO3 river waters. Aragonite and dolomite contents increase away from the springs and toward the river waters that uniquely reflects the effect of Mg ions on the precipitation of aragonite versus calcite. Other potential factors controlling the precipitation of these CaCO3 polymorphs are the Mg/Ca ratio, the CO2 content, and the temperature of the fluids. Dolomite forms during lithification of travertine due to periodic flooding of river water combined with subsequent evaporation.