Gan Zhang, Juan Morales and Juan Manuel García-Ruiz, J. Mater. Chem. B, 2017, 5, 1658.
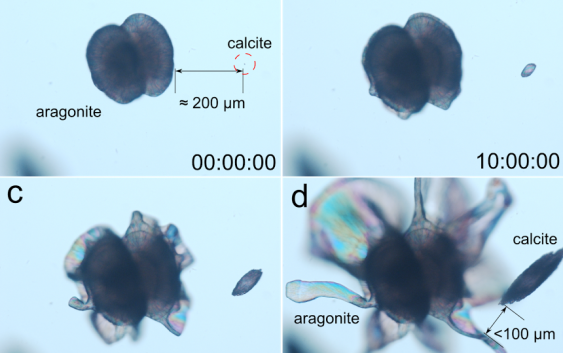
The precipitation of barium and strontium carbonate in alkaline silica gels or silica solutions produces nanocrystalline self-assembled composite materials displaying biomimetic shapes and textures. We have crystallized concomitantly in time and space two anhydrous polymorphs of calcium carbonate, under similar conditions at different temperatures. The orthorhombic phase aragonite produces nanocrystalline aggregates exhibiting non-crystallographic morphologies and complex textures characteristic of silica biomorphs. Conversely, the simultaneously forming trigonal phase, calcite, yields rhombohedral crystals that experience fibrous growth and that maintain memory of the point symmetry group of the crystalline structure. Experiments performed at different temperatures (room temperature, 45, 60 and 80 1C) revealed calcite was never fully inhibited. We have studied the growth mechanism, the growth texture and the that the higher the temperature the higher the aragonite/calcite precipitation ratio, but the crystallization of morphogenesis for both cases. We have found that the dramatic difference between the crystallization behaviours of the two mineral phases is due to the difference in the growth mechanism at the nanoscale.