Giampouras, M., Garrido, C. J., Bach, W., Los, C., Fussmann, D., Monien, P., & García-Ruiz, J. M. (2020). On the controls of mineral assemblages and textures in alkaline springs, Samail Ophiolite, Oman. Chemical Geology, 533, 119435.
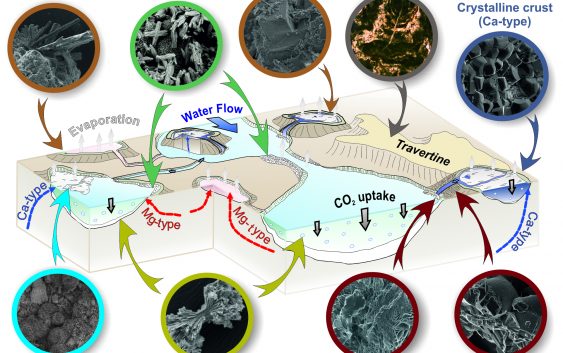
Interactions between meteoric water and ultramafic rocks in the Oman Ophiolite generate waters of variable physicochemical characteristics. The discharge of these waters forms complex alkaline pool networks, in which mineral precipitation is triggered by mixing, evaporation, and uptake of atmospheric CO2. A systematic and co-localized sampling of waters and solids in two individual spring sites allowed us to determine the saturation state of a range of minerals and correlate them to the different water and precipitate types. We subdivided the waters of the spring sites into three distinctive types: i) Mg-type; moderately alkaline (7.9<pH<9.5), Mg2+–HCO3−-rich waters, ii) Ca-type; hyperalkaline (pH>11.6), Ca2+–OH−-rich waters, and iii) Mix-type; alkaline to hyperalkaline (9.6 < pH < 11.5) waters with intermediate chemical composition. We first report the occurrence of hydrated magnesium (hydroxy-) carbonate phases in Mg-type waters. Nesquehonite forms in these waters via evaporation and transforms into dypingite and hydromagnesite under CO2-rich conditions. In Ca-type waters, the coupling of atmospheric CO2 uptake with evaporation leads to the formation of a calcitic crystalline crust on the air-water interface. The crusts are aragonite- and brucite-bearing, where Mg-type and Ca-type waters discharge and vigorously mix at the same pool. Unlike the Mg-type and Ca-type waters, the pools of Mix-type waters host massive aragonite-dominated deposits due to high Mg/Ca ratio that favors the growth of aragonite over calcite. The hydrodynamics during mixing spatially control brucite precipitation and restrict its formation and accumulation around specific mixing zones, where a continuous supply of Mg of inflowing Mg-type waters takes place. Crystal morphologies record the effect on the values of supersaturation and supersaturation rates in the pools due to mixing processes, evaporation and CO2 uptake. In Ca-type waters, CO2 uptake and evaporation dictate the textural characteristics of calcite both in crystalline crusts and rock coatings. Textural evolution of aragonite from crystalline sheaves to spheroidal shapes underlines the different supersaturation rates of calcium carbonate crystallization in flocculent material of Mix-type waters. Geochemical models of mixing between Mg-type and Ca-type waters revealed the evolution of mineral saturation indices under various mixing proportions, and their relation to the observed mineralogy and geochemistry of the pool waters. The thorough documentation of mineral assemblages and crystal morphologies enabled us to provide a more detailed account of how water composition, mixing, and mineral precipitation co-evolve in the alkaline spring systems, where CO2 is sequestered.